Clear or Amber USP Type 1 borosilicate sterile glass vials with a standard 20mm crimp finish (13mm hole opening x 20mm outer rim diameter). Outside diameter x height: 22x40mm
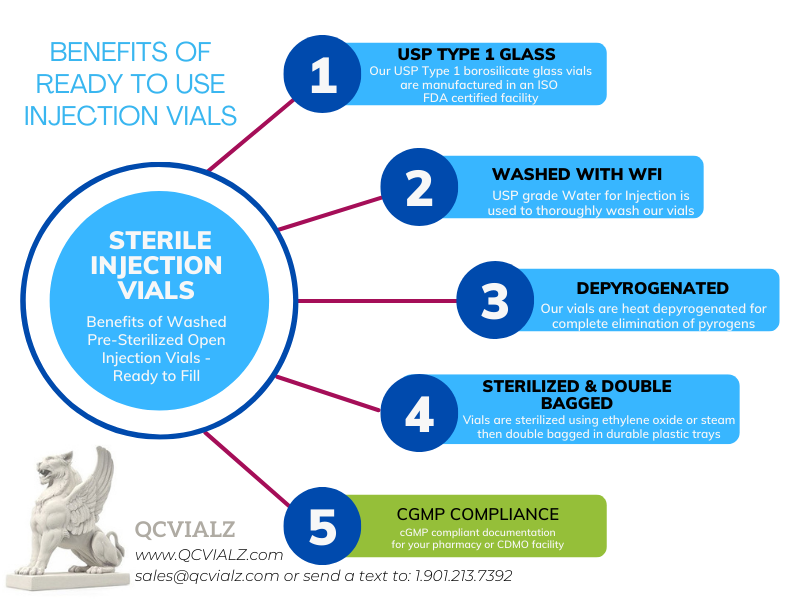
USP Type 1 borosilicate glass vials are formed and then washed using USP Water for Injection (WFI) by Gerresheimer®. The washed vials are depyrogenated using heat, and then sterilized using ethylene oxide by SteriGenics®. The finished vials are arranged in durable nested plastic trays and hermetically sealed.
NESTED TRAYS: unique nested tray (alveolar style tray) keeps glass vials from touching one another during transit and storage. This designs greatly reduces the chances of scratches or damages. The nested tray can also serve as a sterile vial rack during the filling process in your cleanroom.
With each paid order, IVPACKS customers will receive a certificate of conformance will be prepared. The certificate of conformance will contain the following elements:
▪ EtO residual determination, ISO 10993-7, Current Edition.
▪ Sterilization process conformance, ISO 11135, Current Edition.
▪ Sterility testing conformance, USP <71>, Current Edition
▪ Bacterial endotoxins, NMT 0.125 EU/mL, USP <85>, Current Edition
▪ Commercial information (order, date of production, quantity, glass tubing, etcetera).
Single sealed nested tray of 96 pieces